Overview
What is a plasma?
The name plasma denominates a partly ionized gas. Plasmas also appear in nature, for example in lightnings. Plasma is also often called »the fourth state of matter« because if energy is applied to a solid it becomes liquid. If more energy is applied it becomes gaseous and eventually it becomes a plasma.


How is a plasma created?
A chamber is filled with a defined volume of gas. The energy to create a plasma is applied by an oscillating electromagnetic field. Depending on the application the oscillation frequency is in the range of MHz, GHz or kHz.

Structure of a Typical Plasma System
A low-pressure plasma system essentially consists of the following main components: a vacuum chamber, a vacuum pump, a gas distribution system, a plasma generator with an electrode system, and a control cabinet with a computer.
The process for cleaning, activating, or coating components is carried out in several steps:
- Loading the components: The components to be processed are placed into the vacuum chamber.
- Evacuating the chamber: The air inside the chamber is evacuated using a vacuum pump until the preset base pressure (desired vacuum) is reached.
- Introducing the process gases: Once the base pressure has been achieved, specific process gases are introduced into the chamber.
- Plasma generation: The introduced process gases are ionized by the plasma generator and the electrode system, creating plasma. During the process, the process gas is continuously evacuated via the vacuum pump. This ensures a constant supply of fresh process gases while efficiently removing any by-products generated during the process.
- Processing the components: Depending on the process selected, the components are cleaned, activated, and/or coated within the plasma.
- Ending the process: After the preset process duration, the generator, gas supply, and evacuation system are stopped. The vacuum chamber is then vented via a ventilation valve.
- Removing the components: The processed components can now be removed from the chamber.
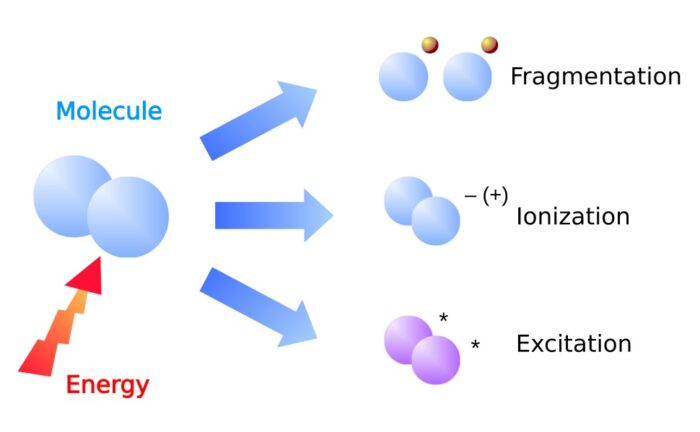
What happens in a plasma?
In a plasma 3 basic effects occur parallel:
- Fragmentation – chemical bonds in the gas molecules are cracked resulting in highly reactive radicals.
- Ionization – electrons are removed from the electron shell of atoms. The resulting free electrons and ions are reactive.
- Excitation – electrons are lifted up to a higher energy level in the electron shell. After a short time they fall back to the original energy level. The released energy is emitted as electromagnetic radiation.

How are plasmas used?
The excitation is for example used in fluorescent lamps.
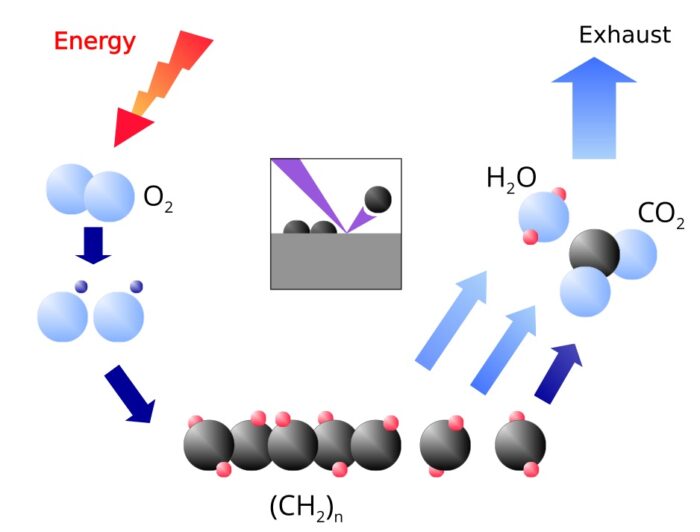
The ionization and fragmentation can be used for the following applications:
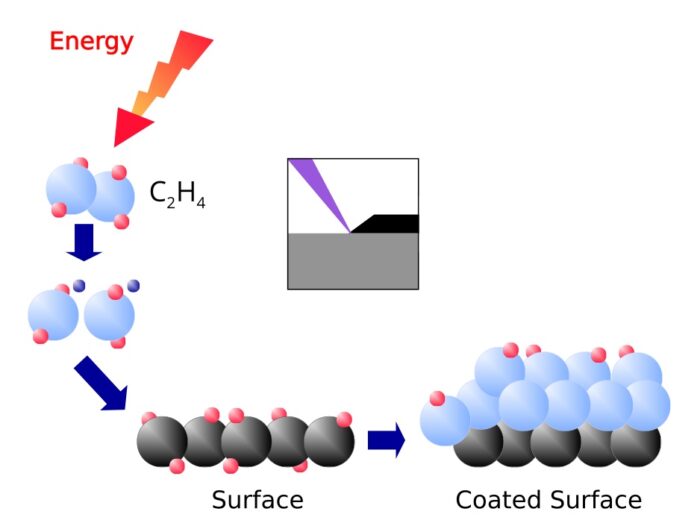
Coating
The radicals and ions of the plasma react with the molecules in the surface. One gets therefore a chemical bond between the surface and the coating. The coating is created by the attachment of further radicals and ions from the plasma.
Plasma is used for the coating techniques PECVD, PEALD and Sputtering.

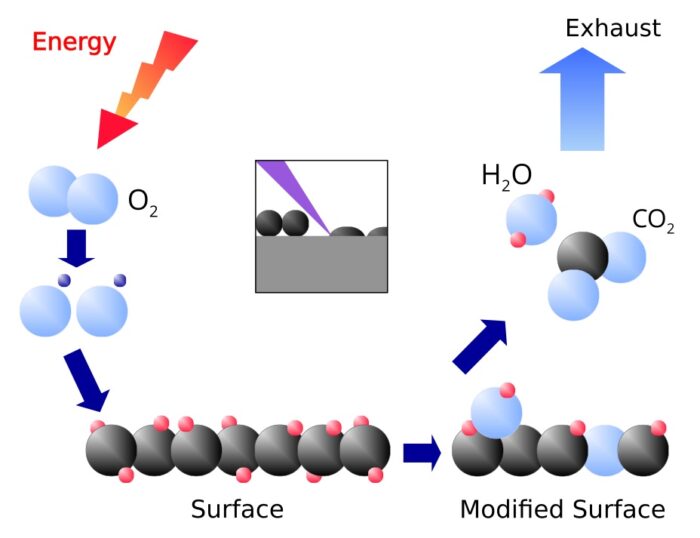
Adhesion promotion
With a plasma treatment it is possible to achieve an optimal adhesion between two materials at the whole interface. The adhesion is provided by forming chemical covalent bonds at temperatures below 50 °C. Plasma treatments create either reactive coatings on the surface or chemically functional groups and radicals in the surface. This allows to connect metals chemically with plastics as well as plastics with plastics. For more details, see this page.